The pharmaceutical industry is under immense pressure to ensure patient and drug safety while dealing with a complex web of global & national regulations. Alarmingly, the World Health Organization estimates that approximately 10% of medicines in low and middle-income countries are counterfeit or substandard. This statistic emphasizes the urgent need for effective track and trace solutions to protect public health and maintain the integrity of the pharmaceutical supply chain.
Without real-time tracking and tracing, companies can suffer from significant supply chain disruptions. The absence of a robust track and trace system can lead to high product duplication, tampering, and diversions. Poor tracking can result in errors, wastage, and a loss of brand perception, while also increasing the chances of wrong inventory allocation and mismanagement. Additionally, a lack of automation often results in cumbersome paperwork, increased costs, and human errors.
What is Track and Trace in Pharma?
Track and trace solutions in the pharmaceutical sector are designed to monitor the movement of drugs throughout the supply chain. By assigning unique identifiers to each product, these systems provide transparency and security, allowing stakeholders to verify the authenticity of medications at every stage—from production to delivery.
The primary objectives of these systems include:
- Preventing Counterfeit Drugs: By tracking each drug’s journey, stakeholders can identify and eliminate counterfeit products.
- Enhancing Supply Chain Visibility: Real-time data allows for better inventory management and quicker responses to potential issues.
- Ensuring Regulatory Compliance: Many countries have stringent regulations requiring detailed tracking of pharmaceuticals to ensure safety.
How Do Pharmaceutical Track and Trace Systems Work?
Pharmaceutical track and trace systems utilize technologies such as barcodes and 2D Data Matrix codes (QR Codes). Each drug package is assigned a unique serial number, which is encoded into a scannable format. This allows for efficient tracking throughout its lifecycle.
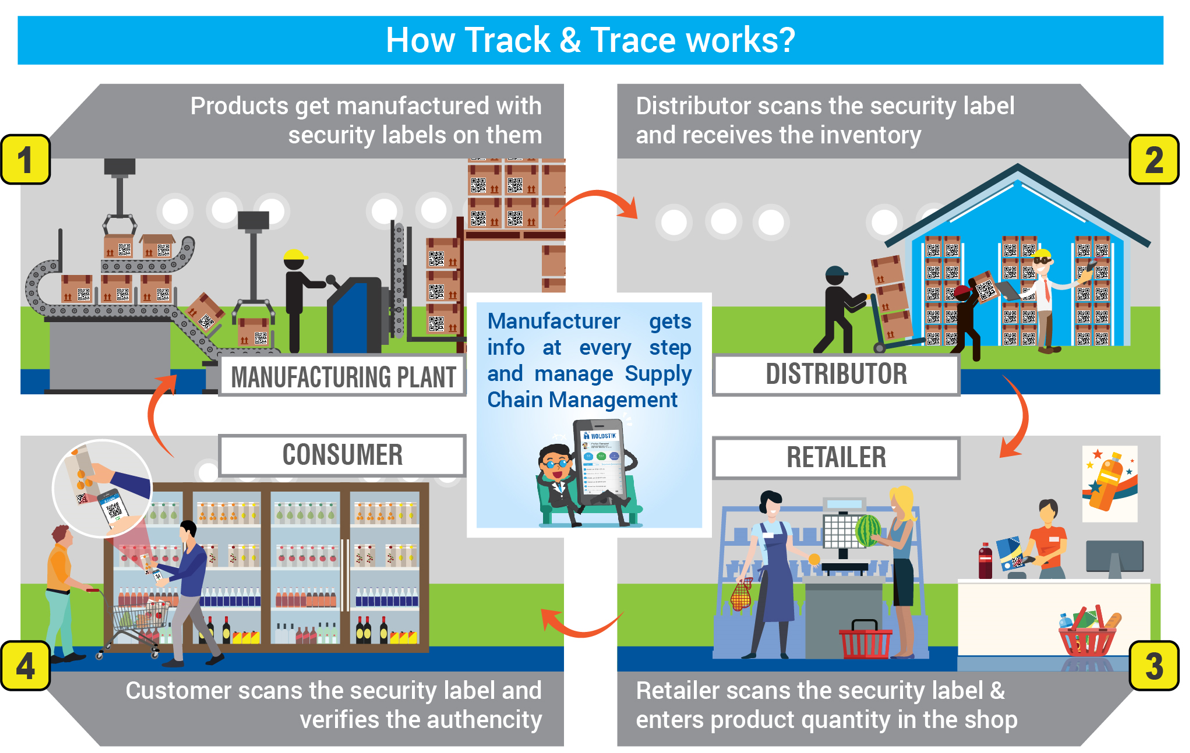
Key components include:
- Unique Identifiers: Each drug box has a digital ID that contains critical information such as the Global Trade Item Number (GTIN), expiration date, serialization number, and batch number.
- Real-Time Data Capture: Scanners throughout the supply chain capture these codes, feeding real-time data into centralized databases for monitoring and analysis.
- Regulatory Compliance: Systems must adhere to regulations of Central Drugs Standard Control Organization (CDSCO).
Challenges in Implementing Track and Trace Solutions
While the benefits of track and trace systems are substantial, several challenges hinder their widespread adoption:
- Supply Chain Complexity: The pharmaceutical supply chain involves multiple stakeholders—manufacturers, distributors, retailers—which complicates tracking efforts.
- Interoperability Issues: Different countries may have varying standards for serialization, making it difficult to achieve seamless integration across borders.
- Technological Barriers: Implementing advanced tracking technologies can be costly and complex, requiring significant investment in infrastructure.
- Stakeholder Resistance: Some stakeholders may be reluctant to adopt new technologies due to concerns about costs or disruptions to existing processes.
Pharma Track and Trace Solutions
Utopia Digitech, the digital arm of Holostik offers a comprehensive track and trace solutions that addresses many challenges faced by pharmaceutical companies today. Their solution prevents product duplication, tampering, and diversion through digital solutions for real-time product authentication and traceability.
Key Benefits of Our Pharmaceutical Track and Trace Solutions
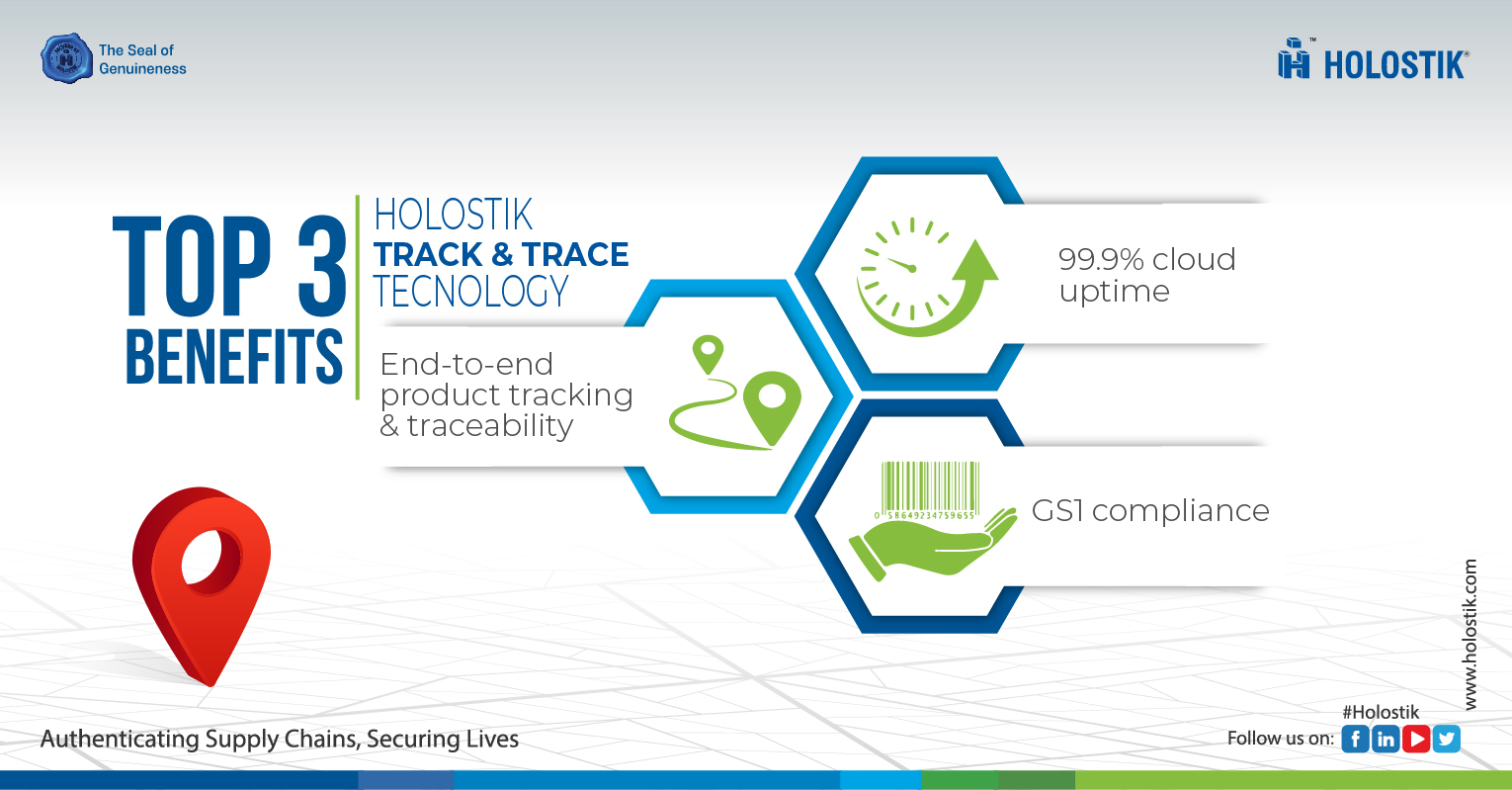
- Mitigation of Product Delays: Their system effectively reduces errors in inventory allocation while ensuring timely delivery of products.
- End-to-End Traceability: Utopia Digitech provides complete visibility throughout the supply chain to prevent disruptions and eliminate information siloes.
- Integration with Third-Party Software: This feature saves time and monetary investment by allowing seamless interaction with existing systems.
- High Cloud Uptime: With a cloud uptime guarantee of 99.9%, users can expect hassle-free services and efficient workload management.
- Regulatory Compliance: The system adheres to GS1 standards, ensuring compliance with international regulatory requirements.
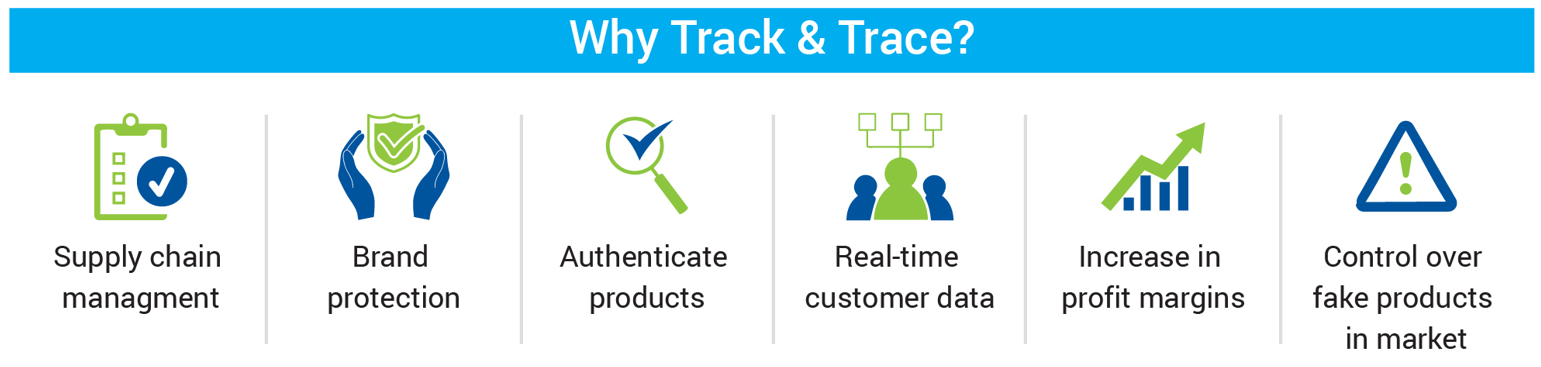
Why Choose Holsotik Track and Trace Solutions?
Holostik stands out as an industry leader due to its high-end Phygital anti-counterfeiting solutions which incorporates custom track & trace solutions through printed QR codes on packaging. Companies utilizing this solution have reported significant business growth due to enhanced operational efficiency.
Additional advantages include:
- CMMI L3 certification ensuring high organizational maturity in software development.
- Custom digital solutions tailored to client-specific needs.
- Extensive experience with over 30 years in optimizing supply chains across various sectors.
- Round-the-clock tech support available through pan India offices for prompt assistance.
The implementation of track and trace solutions is essential for ensuring drug safety in an increasingly complex world. With companies like Holostik leading the way with innovative solutions that enhance transparency, combat counterfeit medications, and ultimately protect patient health, the pharmaceutical industry is better equipped to face its challenges. As technology continues to evolve, these solutions will play a pivotal role in shaping a safer future for pharmaceuticals worldwide.


